To achieve the goal of reducing the carbon footprint of concrete by means of accelerated carbonation, the FastCarb project covers both upstream and downstream aspects. The objective of the upstream part of the project is to validate, from a theoretical and experimental point of view, the technical proof of concept already obtained in the laboratory for the accelerated carbonation of recycled concrete aggregate (RCA).
To achieve these aims, WG1 includes 3 sub-working groups:
Experimental approach
WG1.1 focuses on the experimental approach in the laboratory. It will provide the experimental data needed to analyse the phenomena involved in the accelerated carbonation of recycled concrete aggregate and understand the influence of major factors (water content for example) in order to model the process. This working group covers 3 interdependent areas:

Figure 1. The different areas covered by WG1.1
Area 1: Development of the carbonation procedure for RCA
Area 2: Understanding the mechanism of accelerated carbonation
Area 3: Changes in the physico-chemical properties of RCA
Two of the areas covered by WG1.1 have already been completed. Area 1 relates to the development of the laboratory procedure for the accelerated carbonation of the recycled aggregate (RCA). Three procedures were developed in different laboratories:
Accelerated carbonation of RCA (static test directed by Marie Sereng):

Figure 2. The static accelerated carbonation process for RCA
Presaturation of the RCA
RCA placed in a desiccator
Injection of CO2 for 24 hours (at atmospheric pressure)
RCA dried at 90°C
Accelerated carbonation of RCA in a rotating drum (test directed by Bogdan Cazacliu):
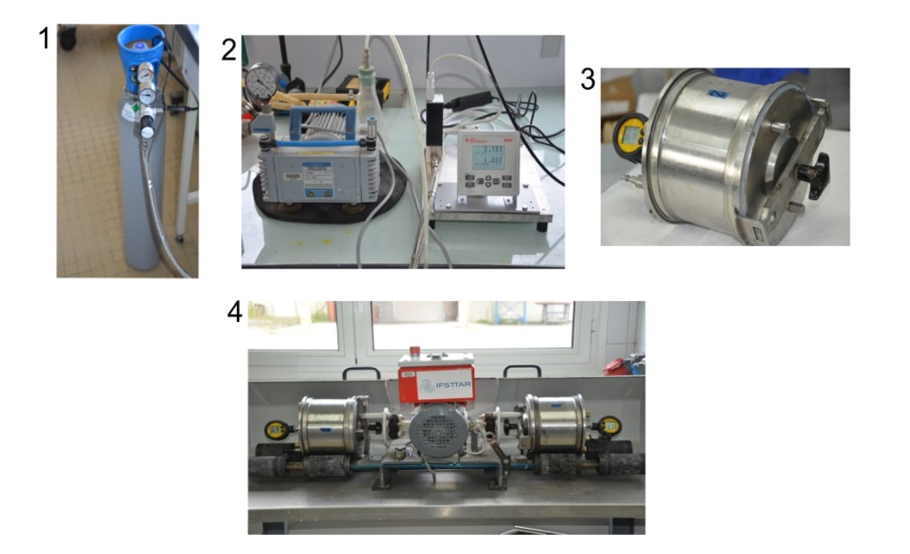
The setup for the laboratory scale rotating drum carbonation experiments. (1) CO2 gas cylinder; (2) automatic pump vacuum and CO2 flow regulators; (3) sealed container for the aggregate (with pressure sensor); (4) rotating drum system.
Accelerated carbonation of RCA in a multi-constituent gas containing, CO2, SO2 and NOx (test directed by Jena Jeong):

Figure 4. a) Internal view of the accelerated carbonation device b) humidifier
CO2 was supplied from a CO2/air mixture cylinder (15% of CO2 for carbonation with pure CO2) and from a CO2+SO2+NO2/air mixture cylinder (15% of CO2, 390 ppm of NO2 and 35 ppm of SO2) for carbonation with a multi-constituent gas whose flow rate was controlled by a manometer.
Area 3 is the study of changes in the physico-chemical properties of the RCA, such as changes in the water absorption coefficient, the shape and surface texture of the RCA, and the carbonatable phase content.
Optimization of the parameters (material properties and exposure parameters) for complete carbonation of the demolition RCA:
Optimization of the parameters (material properties and exposure parameters) for complete carbonation of the demolition RCA:
There is an optimal water content for a maximum amount of stored CO2. The initial natural carbonation affects the accelerated carbonation of the RCA and CO2 storage, by altering the carbonatable phase content of the RCA (portlandite for example). 1/4 RCA from beams that has undergone little natural carbonation stores up to 43.6 g/kg, for partially naturally carbonated crushed 1/4 RCA from the national RECYBETON project (hereafter referred to as RB RCA), the CO2 storage rate is 16.3 g/kg. After 6 years of storage, completely naturally carbonated 1/4 RB RCA stores up to 8.6 g/kg (Figure 5).
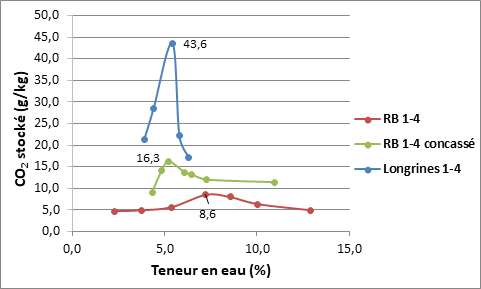
Figure 5. Effect of the initial natural carbonation of RCA on CO2 storage (Marie Sereng’s results)
CO2 stocké : Stored CO2
Teneur en eau : Water content
Carbonation in a rotating drum contributes to the selective attrition of the concrete chippings. The process accelerates carbonation, eliminating carbonated zones on the surface of coarse aggregate particles and then facilitating the access of CO2 to the non-carbonated zones. The dynamic process strongly increased (by a factor of about 6) the carbonation kinetics compared to static carbonation of the same duration (Figure 6). The number of rotations is an important parameter of the process for explaining the increase in carbonation kinetics. However, carbonation time should also be taken into account, independently of the number of rotations.
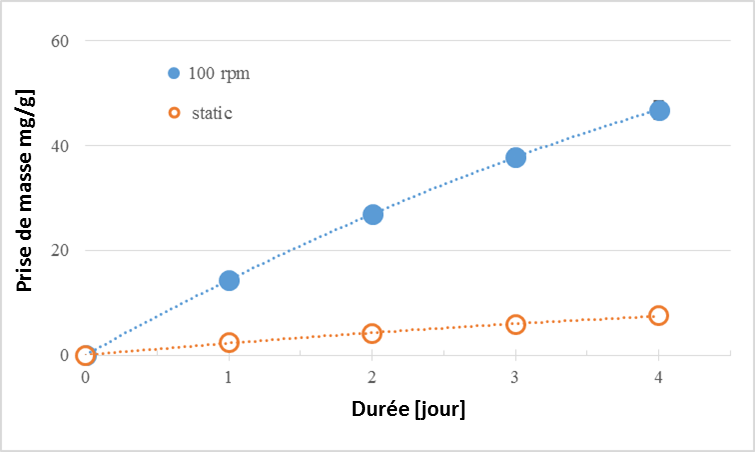
Figure 6. CO2 storage kinetics in static mode and at 100 rpm for recycled chippings (moisture content ranging from 7.3 to 7.6%) (Glaydson Simoes’ results)
Increase in mass (mg/g)
Duration (days)
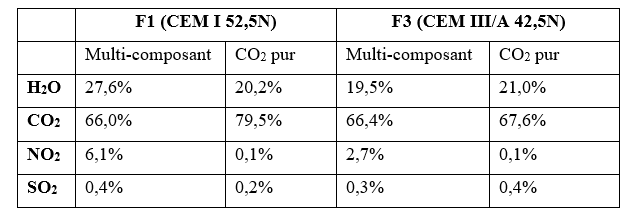
In the case of the accelerated carbonation of RCA in the presence of the multi-constituent gas, the NO2 and SO2 are well adsorbed and/or precipitated on the amorphous CaCO3. With regard to the concentration of NO2 in the mixture, a significant amount of NO2 trapped during the precipitation of calcium carbonates was measured. This could explain why less CO2 is trapped in the case of the multi-constituent gas than in the case of pure CO2. This phenomenon is similar for both F1 and F3 (Table 1).
Effect of accelerated carbonation on the properties of RCA: Accelerated carbonation lowers the coefficient of water absorption as pores are filled by various forms of CaCO3 deposited during the carbonation reaction. Accelerated carbonation lowers the coefficient of absorption on the crushed fraction (1/4) of RB RCA by 65%, while over a 6-year period natural carbonation decreases the coefficient of water absorption of 1/4 RB RCA project by 53% (Figure 7).
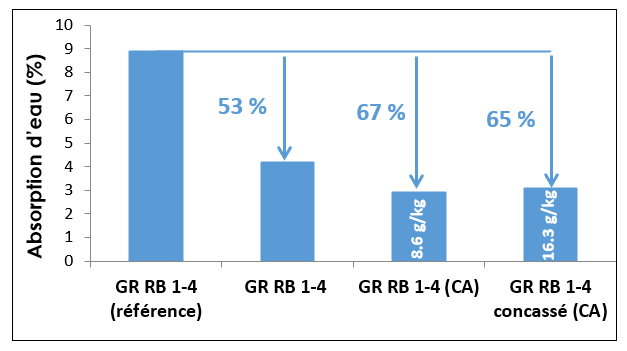
Figure 7. Effect of natural and accelerated carbonation on the coefficient of water absorption (results from M. Sereng)
Water absorption (%)
1/4 RB RC (control) ;1/4 RB RC ; 1/4 RB RC ; crushed 1/4 RB RC
The rotating drum process improves the shape and surface texture of the RCA. Before undergoing the process, the aggregate particles had irregular shapes and rough surfaces (see Figures 8A and C). However, after the process, the same aggregate particles were more spherical with smooth surface textures (see Figures 8B and D).
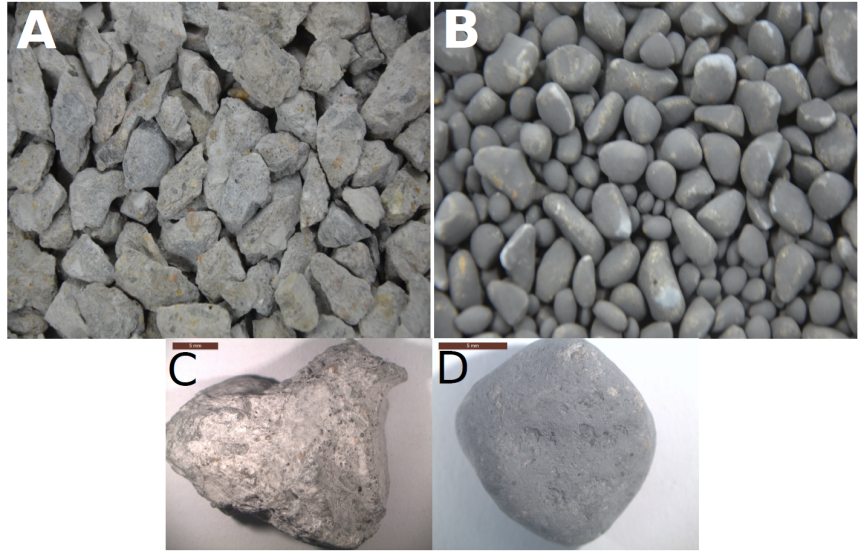
Figure 8. Appearance of the aggregate grains before (A, C) and after (B, D) carbonation (results from Glaydson Simoes)
The nature of the mineral phases formed during accelerated carbonation depends on the type of gas. The precipitation of amorphous CaCO3 was readily observed in all types of samples in the case of pure CO2. Indeed, most of the CaCO3 was present in amorphous form as we would have expected more intense peaks if the CaCO3 had been fully crystallized. Moreover this was difficult to identify in the case of the multi-constituent gas. The presence of Vaterite was also identified for F3 with pure CO2, but this was difficult with the multi-constituent gas (Figure 9).
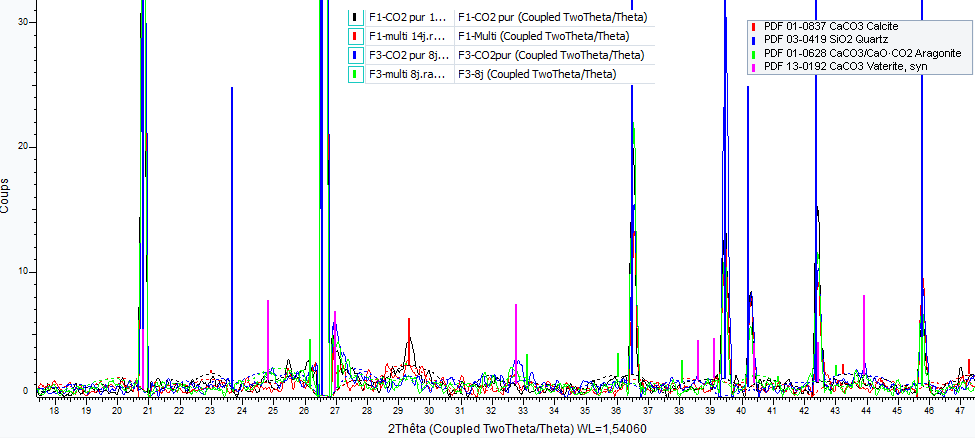
Figure 9. Identification of mineral phases by X-ray diffraction for F1 after 14 days and F3 after 8 days (results from Jena Jeong)
Modelling
WG1.2 deals with the modelling of carbonation and adds to the experimental studies of WG1.1 by providing a degree of understanding of the phenomena involved in the fixation of CO2 by Recycled Concrete Aggregate (RCA). Such investigation is complex because it uses parameters that are sometimes difficult to measure experimentally in view of the small size of the RCA particles, such as the spatial distribution of the carbonates formed.
WG1.2 is also concerned with the natural carbonation of concrete during the life of the structure, as this can have a significant impact on the carbon footprint of the material. The aim is to provide insights into the role of natural carbonation when assessing the contribution of construction works to sustainable development, with reference to the standard NF EN 16757 “Sustainability of construction works. Environmental product declarations. Product Category Rules for concrete and concrete elements” and the documentation booklet CEN/TR 17310 “Carbonation and CO2 absorption in concrete”.
WG1.2 consists of several sub-tasks.
Benchmarking:
The partners’ carbonation models were benchmarked on the basis of experimental data taken from the literature (Figure 1). This highlighted the advantages and limitations of each model. Analysis of the results allowed us to identify ways of improving the models – which were initially developed to study the carbonation of concrete from the point of view of the durability of structures – in order to apply them to the study of CO2 fixation by the RCA.
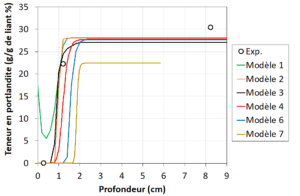
Figure 1. Result of benchmarking: comparison of the portlandite content profiles obtained from 6 carbonation models and the experimentally determined contents (Exp.) after 1 year’s accelerated carbonation of a cement paste.
Parametric investigation: (ongoing studies)
The models used in benchmarking were then exploited in numerical simulation campaigns to identify the parameters which have the most influence on the accelerated carbonation of RCA. The modelling was done at the scale of a homogeneous spherical aggregate particle with homogeneous composition (Figure 2). Figure 3 shows, for example, the influence of the size of the RCA particle and its degree of water saturation on the rate of CO2 capture.
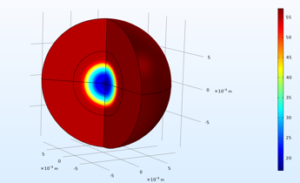
Figure 2. Example of the spatial distribution of the CO2 content fixed by carbonation of a homogeneous spherical RCA particle (CO2 content in g per kg of RCA).
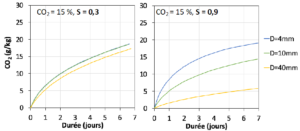
Figure 3. Results of numerical simulations: influence of the diameter (D) and the degree of water saturation (S) of an RCA on the rate of CO2 fixation by accelerated carbonation at an ambient CO2 concentration of 15% (approximately that of cement plant fumes).
Investigation of natural carbonation: (ongoing study)
A reinforced concrete structure is capable of fixing atmospheric CO2 during its working life. As an example (Figure 4), for a typical six-storey building built using CEM II/A, the quantity trapped in 50 years is estimated at 2.2 tonnes, i.e. about 13% of the quantity emitted during the decarbonation of the limestone used to manufacture the cement. This estimate was made using two models: an “engineering” type model (the methodology and assumptions used are compatible with the standard NF EN 16757) and a physico-chemical model (SDReaM-crete developed by CERIB). In addition, these studies will provide input for the databases required for the LCA, and make it possible to assess the initial carbonation state of the RCAs (before accelerated carbonation). The next study will deal with the Aziyadé student hall of residence in La Rochelle.
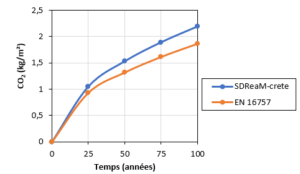
Figure 4. Amount of trapped CO2 per square metre of concrete exposed to atmospheric carbonation in the case of a 6-storey building.
Modelling sustainability: (forthcoming study)
Alongside the experiments conducted by WG2.2, a study is planned to estimate the durability of reinforced concrete structures containing various proportions of carbonated RCA, with regard to two phenomena that attack reinforcement: carbonation (XC) and chloride transfer (XS).
Bio-carbonatation
WG1.3 intends to study an alternative method for fixing atmospheric CO2 on recycled concrete aggregate: bio-carbonation. The objective of this working group is for bacteria to speed up the formation of a clump of CaCO3 around the aggregate particles. This could also improve the performance of the aggregate.
Selection of microbial strains:
Micro-organisms were isolated from recycled concrete aggregate stored outside for several years at the University Gustave Eiffel (formerly IFSTTAR) site in Nantes. About twenty strains were cultured. They were then tested to ensure their ability to (i) grow at pH 11 (which is close to the pH on the surface of the aggregate particles); (ii) precipitate calcium carbonates; and (iii) form a biofilm (an important characteristic to ensure that the bacteria colonize the surface of the aggregate particles homogeneously and that the substances produced as a result of their activity adhere to the aggregate).
These tests resulted in the selection of three strains (Fig. 1) that were candidates for the precipitation of calcium carbonate and its growth on the surface of cementitious materials.

Figure 1. Photographs showing the three selected strains on an agar culture medium in order to observe their capacity to form biofilms.
Placing the strains in contact with mortar:
These strains were put in contact with mortar cylinders. After incubation, the mortars were observed by optical microscopy and scanning electron microscopy. Raman microspectroscopy analyses were also conducted in order to characterise the minerals formed.
The analyses revealed the presence of spheres measuring between 15 – 20µm on the surface of the mortars as well as the presence of bacteria and biofilm (figure 2A and B). The micro-Raman analyses confirmed the presence of calcite and vaterite on the surface of the mortars.
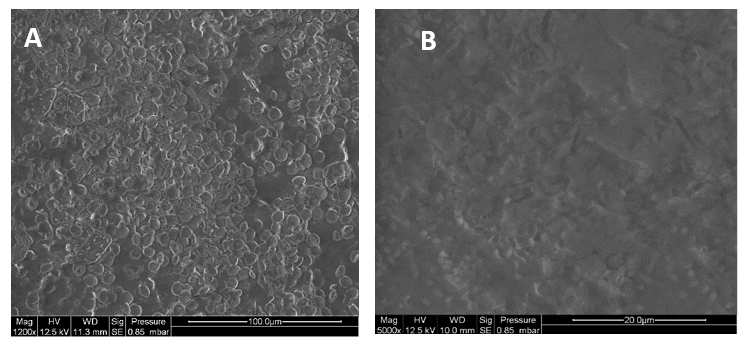
Figure 1. Photographs showing the three selected strains on an agar culture medium in order to observe their capacity to form biofilms.
Treatment of the recycled concrete aggregate:
The next part of the project will focus on treating recycled concrete aggregate and assessing the impact of the activity of the bacteria on its mechanical performance. The ultimate goal will be to assess the material with regard to the amount of atmospheric CO2 it consumes.
